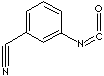
| 3-CYANOPHENYL ISOCYANATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 16413-26-6 |
|
| EINECS NO. | ||
| FORMULA | NCC6H4NCO | |
| MOL WT. | 144.13 | |
|
H.S. CODE |
||
| TOXICITY | ||
| SYNONYMS | 3-Isocyanatobenzonitrile; 3-Isocyanatobenzonitrile; | |
| 3-Isocianatobenzonitrilo; | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | whit yellowish crystalline solid | |
| MELTING POINT | 51 - 54 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
||
|
NFPA RATINGS |
Health: 2; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.413 - 1.415 | |
| FLASH POINT | 32 C | |
| STABILITY | Stable under ordinary conditions. Moisture sensitive | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
Cyanic acid
(the isomer of fulminic acid) is an unstable (explosive), poisonous, volatile,
clear liquid with the structure of H-O-C≡N (the oxoacid formed from the
pseudohalogen cyanide), which is readily converted to
cyamelide and fulminic
acid. There is another isomeric cyanic acid with the
structure of H-N=C=O, called isocyanic acid. Cyanate group (and isocyanate group) can react with itself.
Cyanuric acid (also called pyrolithic acid), white monoclinic crystal with
the structure of [HOC(NCOH)2N], is the trimer
of cyanic acid. The
trimer
of isocyanic acid
is called biuret.
Cyanic acid hydrolyses to ammonia and carbon dioxide in water. The salts and esters of cyanic acid are cyanates. But esters of normal cyanic acid are not known. The salts and esters of isocyanic acid are isocyanates. The isocyanate group reacts with the hydroxyl functional group to form a urethane linkage. Diisocyanates (or polyisocyanates) are monomers for polyurethane production. Polyurethane is made from a variety of diisocyanates in conjunction with polyether and polyester polyols as co-reactants by addition polymerization which needs at least two -N=C=O groups. Polyurethanes are widely used in the manufacture of flexible and rigid foams, fibres, coatings, and elastomers. If isocyanate monomer is polymerized with amine group, polyurea is produced. Cyanates (or Isocyanates) are readily reacts with various form of amine (including ammonia, primary-, secondary-amines, amides and ureas) and hydroxyl functional group. They are used in the synthesis for the target molecules such as pharmaceuticals, pesticides, textile softener, lubricants and industrial disinfectants. They can convert to polycyclic compounds such as hydantoins and imidazolons. They are used as plastic additives and as heat treatment salt formulations for metals. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
whit yellowish crystalline solid | |
| ASSAY | 98.5% min | |
| MELTING POINT | 51 - 54 C | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | 6.1 (Packing Group: III) | |
| UN NO. | 2206 | |
| OTHER INFORMATION | ||
| Hazard Symbols: XN, Risk Phrases: 20/21/22-36/37/38, Safety Phrases: 7-26-27-37/39 | ||
| GENERAL DESCRIPTION OF BENZONITRILE |
||
| Nitrile is an
organic compounds containing cyano group (-C≡N, containing trivalent nitrogen)
which is attached to one carbon atom with the general formula RC≡N. Their names
are corresponding to carboxylic acids by changing '-ic acid' to '-onitrile', or
'-nitrile', whichever preserves a single letter O. Examples are acetonitrile
from acetic acid and benzonitrile from benzoic acid. The prefix cyano is used as
an alternative naming system to indicate the presence of a nitrile group in a
molecule. Sodium cyanide, NaCN; potassium cyanide, KCN; calcium cyanide,
Ca(CN)2; and hydrocyanic (or
prussic) acid, HCN are examples. Chemically, the simple inorganic cyanides
resemble chlorides in many ways. Organic nitriles are used as;
·
Extraction solvent for fatty acids, oils and unsaturated hydrocarbons Benzonitrile, derived mainly from benzoic acid reaction with lead thiocyanate by heating, is a clear liquid; boils at 191 C. It reacts violently with strong acids to produce toxic hydrogen cyanide. It decomposes on heating producing very toxic fumes, hydrogen cyanide, nitrous oxides. Benzonitrile is used as a solvent and chemical intermediate for the synthesis of pharmaceuticals, dyestuffs and rubber chemicals through the reactions of alkylation, condensation, esterification, hydrolysis, halogenation or nitration. Benzonitrile and its derivatives are used in the manufacture of lacquers, polymers and anhydrous metallic salts as well as intermediates for pharmaceuticals, agrochemicals, and other organic chemicals. |
||